Table Of Contents
- Options for Stage II Melanoma: Making the Decision That’s Right For You
- What is Stage II melanoma?
- Is Stage II melanoma at high risk for coming back (recurring)?
- It is true that Stage II melanoma can have a worse prognosis than Stage III melanoma?
- How can I lower the risk of Stage II melanoma coming back? What adjuvant drugs are used?
- What are the side effects of adjuvant therapy?
- How is adjuvant therapy given?
- How do you weigh the benefits and risks of adjuvant therapy vs active surveillance?
- What do you tell patients who have Stage IIA melanoma and are worried about their risk for recurrence?
Dr. Geoffrey Lim: Can you please tell us about the studies you conducted looking at therapies to reduce the risk of melanoma coming back?
Dr. Jason Luke: Yes. Now, let’s go over some of the details from the clinical trial that established the use of pembrolizumab in Stage II melanoma. Pembrolizumab is an immunotherapy drug that’s been approved by the FDA for many kinds of cancers for a long time, and it was approved for use in Stage III melanoma and Stage IV melanoma.
Given our conversation regarding the risk of melanoma coming back after surgery is similar or perhaps worse for patients with Stage IIB and Stage IIC compared to Stage IIIA and IIIB, it made sense to study whether immunotherapy could provide a benefit in Stage IIB and IIC as well. That led us to do a clinical trial, the Keynote-716 study discussed below.
I also should mention that until recently, outside of clinical trials, all we had to offer these patients with Stage IIB or Stage IIC melanoma was active surveillance. That typically meant looking at the skin and examining the lymph nodes on a regular basis (typically every 3 to 6 months) for up to 5 years after surgery. We were looking for any signs the melanoma had recurred. Sometimes that involved imaging tests as well, particularly if the patient had a suspicious symptom.
Keynote-716 Trial (Pembrolizumab)
In the KEYNOTE-716 trial, patients with Stage IIB and IIC melanoma were split into two groups. Half the patients either had standard follow-up, which would be no treatment (placebo plus an enhanced form of active surveillance), and half the patients received pembrolizumab and an enhanced form of active surveillance. Pembrolizumab was given every three weeks for a year in the same way as it is approved for Stage III melanoma.
As we predicted in designing the study, as soon as we started looking at the two groups at one year of follow-up, we already saw that there was a difference. In other words, pembrolizumab was reducing the recurrences. To describe this numerically, at around 14 months of follow-up, 17% of the patients in the placebo group who had gotten no treatment had a recurrence, and 11% of pembrolizumab-treated patients had experienced a recurrence. That calculates out to a 35% reduction if you do the math.
With longer follow-up, we have continued to see that this reduction in melanoma recurrence has been very stable. We’ve now followed those data out past two years. At a follow-up around 21 months, 15% of the patients in the pembrolizumab group and 24% in the placebo group had their disease come back or had died, which is a 38% reduction with pembrolizumab (Graphic). Based on the patterns we saw over time with pembrolizumab in Stage III patients, we anticipate over time, that we might see approximately a 40% reduction in risk for Stage II patients.
At the time of this writing (March 2023), we’re coming up now to almost 3 years. And that risk reduction has been very, very stable out over time. So, we feel confident telling patients this is the potential benefit that we see.
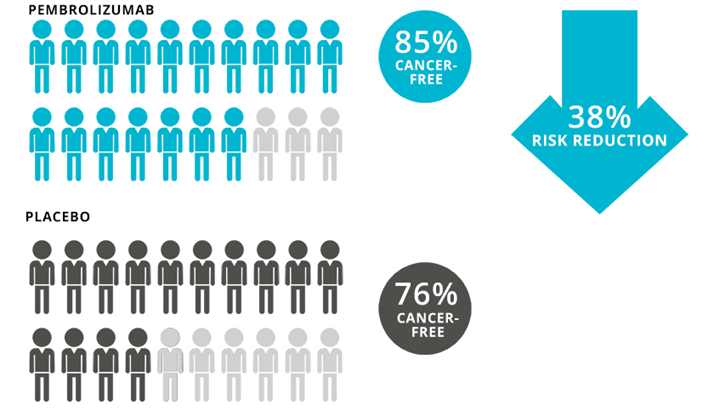
Graphic. Results of the trial of adjuvant pembrolizumab vs placebo in patients with Stage IIB or Stage IIC melanoma at 2 years. To learn more about these data, click here.
CheckMate-76K Study (Nivolumab)
Dr. Jason Luke: I think I’d be remiss if I didn’t also note that there’s another medicine that has shown a similar benefit. That drug is nivolumab, which is also approved for Stage III and Stage IV melanoma. That drug underwent a clinical trial called CheckMate-76K in patients with Stage IIB and IIC melanoma. Preliminary results of this study can be found here. The FDA is formally evaluating it right now for Stage II, but at the time of this writing it’s not yet available. It’s expected to be approved by the end of 2023 as the reduction in recurrence of melanoma was very similar to what we discussed earlier.
Functionally speaking, nivolumab shows a very similar impact on recurrence. That’s what we’ve seen in all other settings of melanoma. So, we assume that soon both of these drugs, nivolumab in addition to pembrolizumab, will likely be available for patients. They have almost identical outcomes and side effects.
And once nivolumab is approved, you might receive either drug. Most likely, the choice will be driven by how much your doctor knows about each drug, not by the perceived difference in how well the drugs work. But both of these, I think, are worth discussing with patients who are facing Stage IIB or Stage IIC melanoma to try to understand, again, the pros and the cons of whether a treatment would be useful to them.
Targeted therapies, which work via a different mechanism in patients with specific mutations in a protein called BRAF, are being studied outside the United States. These therapies were effective as adjuvant for Stage III melanoma, so we look forward to results from the studies in Stage II melanoma as well.
